QuantumScape’s solid-state lithium-metal battery technology is enabled by our proprietary ceramic solid-electrolyte separator. While you may be familiar with everyday examples of ceramics, like a coffee mug, high-tech ceramics are likely less well-known. In this blog, we’ll review the basics of ceramics – what they are, how they’re made, how they’re used – and the relationship between our ceramic material and the broader high-tech ceramics industry.
What is a ceramic?
A ceramic is an inorganic non-metallic solid made up of either metal or non-metal powders that have been blended together and hardened by heating to high temperatures. At the molecular level, ceramics generally have a polycrystalline[1] structure, unlike glass, which has no orderly microscopic structure.[2] There is a vast spectrum of ceramic and hybrid materials, so these should only be considered rules of thumb, but generally, this family of materials shares several characteristics:
- Hard and strong under compression
- Chemically and thermally stable
- Good electrical insulators
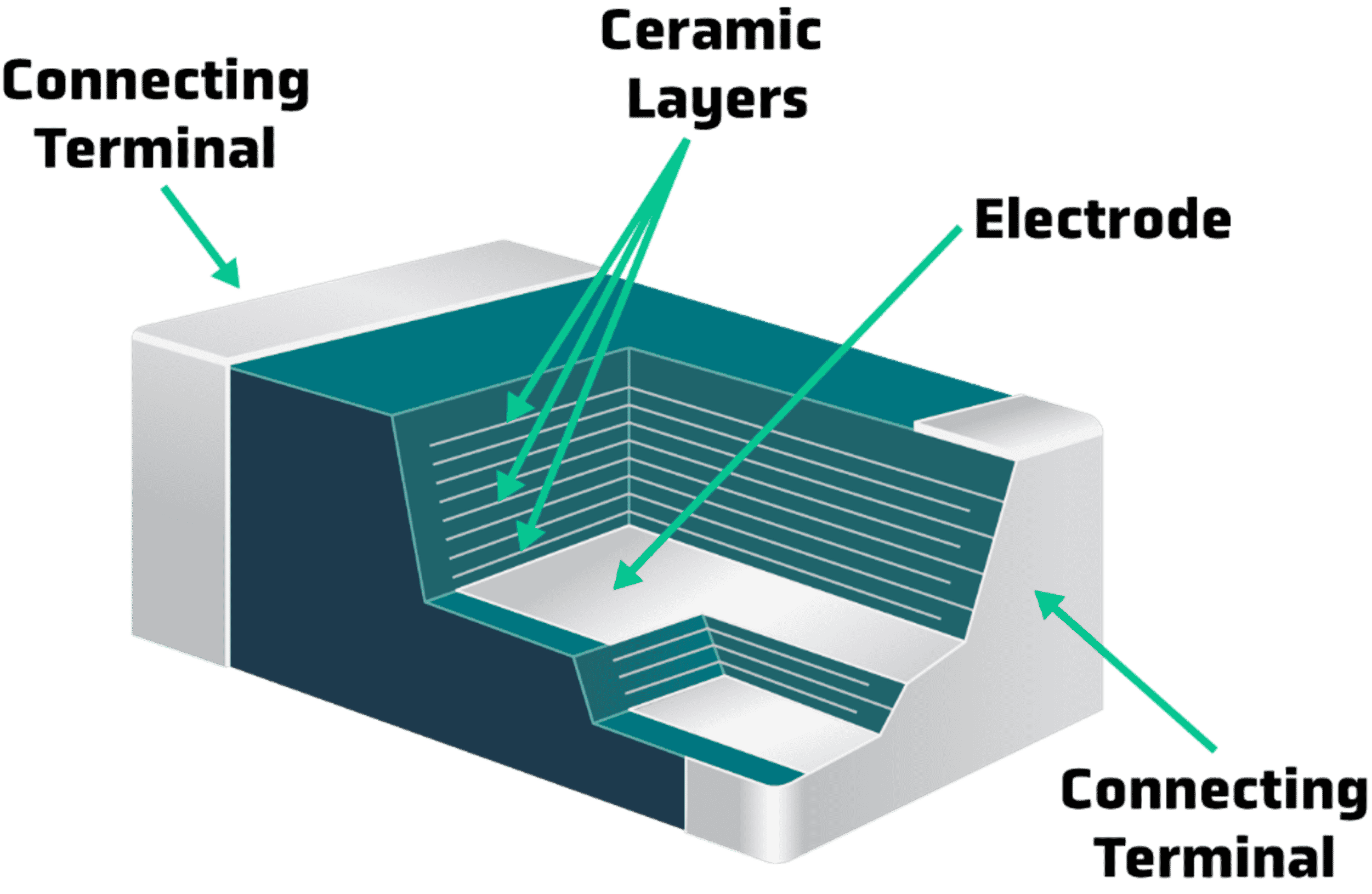
These properties make ceramics attractive for a wide variety of everyday and high-tech applications. For example, specialized ceramics are used for their hardness and compressive strength in dental prosthetics, for their thermal properties in the heat shields on spacecraft, and as insulators in capacitors such as the multilayer ceramic capacitor (MLCC) used for short-term energy storage in devices like smartphones and cars.
Certain properties make ceramics very attractive candidate materials for next-generation batteries. For example, the thermal stability of ceramics means far less risk of combustion or deformation at high temperatures compared to other materials, such as plastics, which soften, melt, and even catch fire under comparatively low heat. These fires are one of the main safety risks of conventional lithium-ion batteries.
Beyond thermal stability, ceramics tend to have excellent hardness, which means they resist deformation, and compressive strength, meaning they are very good at carrying loads. Bricks are a great example of this: they can carry the weight of large buildings without crumbling. At the same time, ceramics are often described as brittle in the technical sense; however, brittle is not synonymous with fragile or inflexible. Brittle means the material doesn’t plastically deform[3] before it breaks, not necessarily that it breaks at a low level of stress.[4]
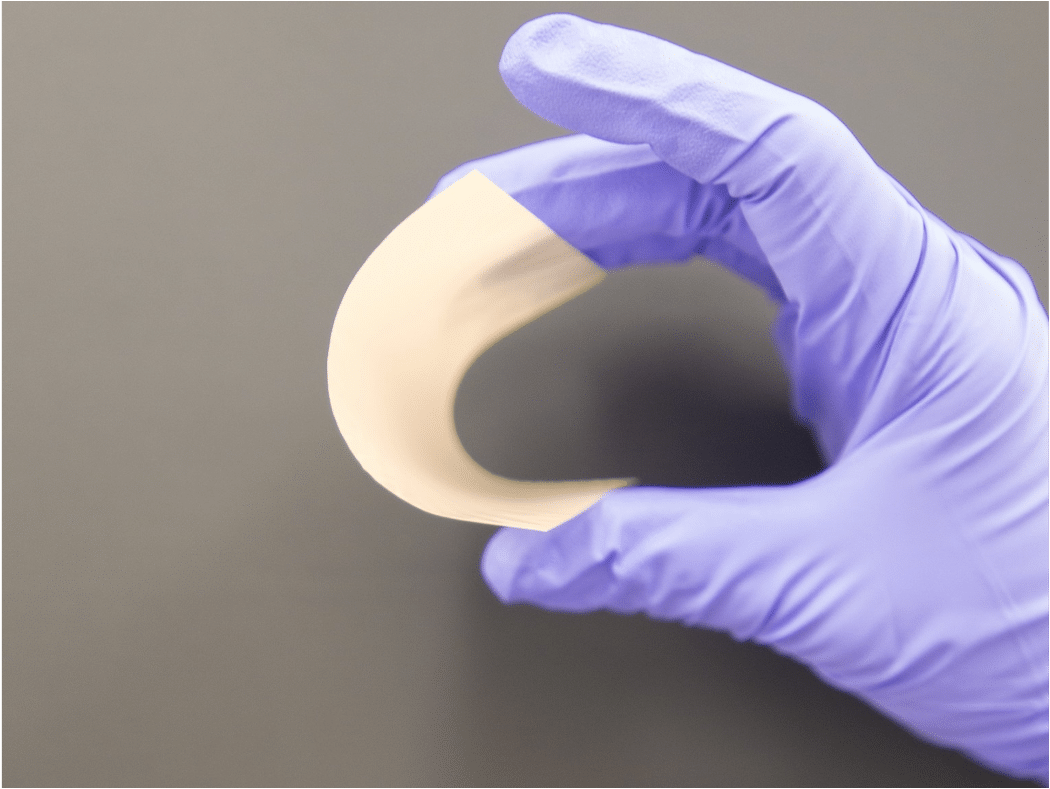
Most everyday ceramics, like a plate or brick, don’t bend at all and will break if you try too hard. But when made extremely thin, ceramics can become flexible enough to bend without reaching a breaking point. This is because the amount of strain the material must withstand when bending is proportional to the distance between the bending surface and the center of the material. For a very thin ceramic, this distance is tiny, which means that the stress it experiences is quite small.
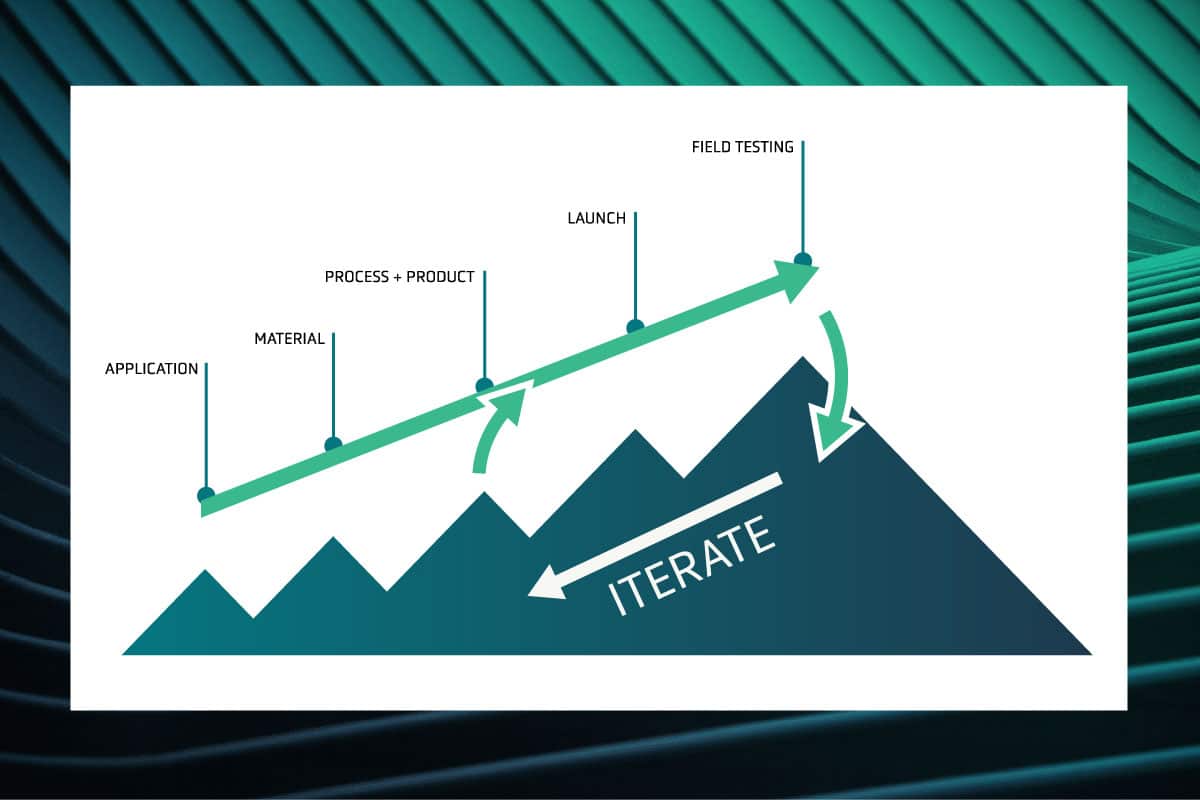
This combination of properties is the key to our battery design and scale-up strategy. On the microscopic scale, the incredible hardness of the ceramic enables it to resist lithium-metal dendrites. At the same time, the macro-scale flexibility of the separator allows it to be handled and processed in a factory setting, which is a requirement for mass production of EV batteries.