
Our Strategic Blueprint
I took on the CEO role last year because I see QuantumScape as the global leader in solid state battery technologies with an ability to revolutionize energy storage and create tremendous shareholder value in the process.
In our blog post on sulfides, we outlined some of the limitations we believe make sulfide-based solid electrolyte separators unsuitable for enabling lithium-metal anode technologies. Here we’ll review the challenges facing two other approaches: liquid electrolytes[1] and solid polymer electrolytes.[2] We group them together because both liquid and solid polymer electrolytes typically consist of organic materials, which give them similar properties.
Lithium metal’s fundamental properties make it the ideal anode material for a lithium-based battery system,[3] as it offers superior energy density (and therefore vehicle range) and can potentially solve the fast-charge bottleneck from which legacy lithium-ion batteries suffer. These benefits explain why battery scientists have been researching the potential for using lithium-metal anodes with liquid and polymer electrolytes since the 1970s.[4] More recently, some polymer-based approaches have even seen limited commercial use. Liquid and polymer electrolytes are superficially attractive technology pathways since they are commonly used in legacy lithium-ion batteries with graphite anodes.
Despite the long history of unsuccessful attempts, new companies continually take up the effort to work with liquids or solid polymers with a lithium-metal anode. But test data we’ve seen[5] shows they still suffer from the same underlying problems — slow rates of charge, poor safety and reliability, and high cost — which is why we believe these efforts will not be commercially viable in vehicles.
The most serious problems that both liquids and solid polymer electrolytes face when paired with high-performance lithium-metal anodes are:
Dendrites are root-like structures of pure lithium that can grow from the anode of a lithium-metal battery cell. In our sulfides article, we describe the dendrite problem in detail. Based on our extensive experience and research on dendrite formation and prevention, we think that neither liquids nor solid polymers can prevent dendrites under the kinds of conditions, such as fast charging rates, required in automotive applications. The reason is simple: the polymer separators in liquid, gel and solid polymer batteries simply aren’t strong enough to resist the growth of these structures, and when the dendrites inevitably reach the cathode, they kill the battery.
And it doesn’t stop there. Dendrites create a short circuit within the cell that can also spark a fire. This is a problem because both liquid and polymer electrolytes are flammable, energy-rich materials, which means a potential fire has plenty of fuel to burn. It’s important to note that safety tests carried out in newly fabricated cells likely won’t show the dramatic impacts on safety that manifest only after the battery has been cycled repeatedly. This is because, as dendrites form, the surface area of the lithium-metal anode increases and makes the cell more susceptible to catastrophic thermal runaway.[6] Not only do such fires pose a risk to driver safety, but EV recalls can cost billions of dollars, potentially making solid polymer and liquid-based systems major liabilities.
Liquids and solid polymer electrolytes haven’t been shown to prevent the destructive effects of dendrites, and this is the main motivator behind the search for a solid electrolyte material that can prevent them. But dendrites are not the only dealbreaker these technologies face.
Liquid and solid polymer electrolytes are inherently unstable and corrode lithium metal,[7] forming chemical junk[8] that increases the internal resistance of the cell. This problem is compounded by dendrite formation: as new dendrites grow, fresh lithium is exposed, creating more junk. Moreover, lithium dendrites can separate from the anode surface, forming clumps of dead material that will further impede the movement of lithium ions. As inactive debris accumulates, it causes a fade in the average voltage of the cell and reduces the energy that the battery can deliver to the vehicle.[9] Importantly, reporting capacity retention of cells with lithium-foil anodes can hide this problem, as the excess of lithium in the foil compensates for the capacity losses, but energy tests of the same cell will show precipitous decline when the cell is cycled. Since energy is what makes a car go, the increased resistance renders such cells unsuitable for use in automotive applications.
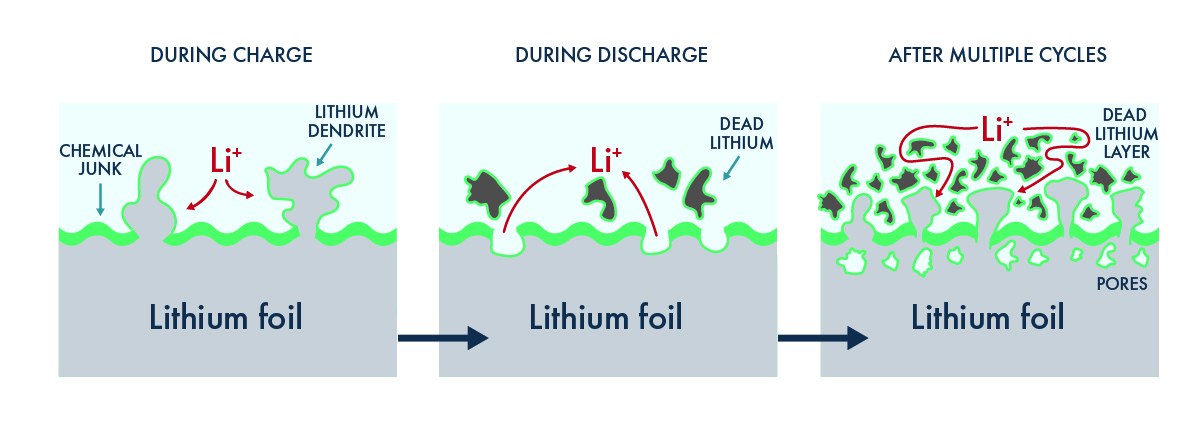
Adapted from J. Mater. Chem. A, 2017,5, 11671-11681
Additionally, solid polymer electrolytes are often poor conductors of lithium ions at room temperature.[10] Commercial solid polymer-based batteries only work at elevated temperatures, such as 60-80 °C. It’s possible to work around this using a very thin layer of polymer on top of a conventional plastic separator filled with liquid electrolyte. Still, approaches like this suffer from issues that combine the worst of both worlds — they insufficiently address dendrite formation and add a layer of material that increases cell resistance.
Finally, the third problem with using solid polymer or liquid electrolytes with a lithium-metal anode: the lithium anode material is typically manufactured first and then built into a cell. For automotive applications, this lithium foil must be made very thin, and because lithium is extremely reactive and difficult to handle, manufacturing at large scales can be cost prohibitive. Adding a coating on the lithium foil further complicates manufacturing. It all adds up, making lithium-metal anode material up to 100x more expensive than typical battery anode materials, potentially doubling the cost of materials per kilowatt-hour and undercutting any potential commercial advantage.[11] And the slightest impurities in the foil accelerate dendrite formation,[12] multiplying the existing performance limitations and safety risks.
Lithium-metal cells using liquids or solid polymers can appear to work when tested under a set of compromised conditions. The workarounds deviate dramatically from real-world conditions. For example:
Our key innovation is a ceramic solid-electrolyte separator, which has allowed us to overcome the fundamental issues that prevent liquid or solid polymer electrolytes from delivering acceptable automotive performance when used with lithium metal. These are decades-old problems: as far back as 2002, Aurbach et al. concluded that, when liquid electrolytes are used, “there is no way that rechargeable [lithium-metal] batteries can compete with lithium-ion batteries in any application that requires high charging rates.”[14]To date, we haven’t seen any data to suggest the problems discussed here have been resolved.
In contrast, we have demonstrated that our solid-state electrolyte can resist dendrite formation at high rates of charge under realistic, automotive-relevant test conditions and is very stable with lithium metal – providing a barrier between the pure lithium anode and our cathode and catholyte materials. Crucially, our separator material also enables the cell to be manufactured anode-free, with the pure lithium-metal anode created during the first charge; this means that our lithium anode material is pure electrochemically distilled lithium metal, created without the cost and complexity involved in handling lithium foil. This combination of properties has allowed us to build a next-generation battery technology that we believe can deliver on the true promise of lithium metal: excellent cycle life, improved energy density, faster-charging speeds, enhanced safety and lower costs.
[1] Traditionally, the electrolyte in a battery — the medium through which lithium ions move inside the cell — is a liquid, typically consisting of an organic compound (e.g., ethylene carbonate, di-methyl carbonate) with a dissolved lithium salt (e.g., LiPF6). The liquid permeates all three major components of a lithium-ion battery: the cathode, anode and separator. The separator is a porous non-conductive plastic film made to keep the anode and cathode electrically isolated. The liquid permeates the pores of the separator and provides a channel for lithium ions to move back and forth between the anode and cathode, which is what happens during a battery’s charge and discharge.
[2] It’s also possible to make organic solids, polymers (e.g., polyethylene oxide) through which lithium ions can flow, albeit at lower rates and higher temperatures. Polyethylene oxide-based batteries have been shown by many players over the years, including UC Berkeley spinout SEEO (acquired by Bosch).
[3] https://doi.org/10.1039/C3EE40795K
[4] https://www.nature.com/articles/nnano.2017.16
[5] https://pubs.acs.org/doi/abs/10.1021/acs.chemrev.7b00115
[6] https://doi.org/10.1016/0167-2738(94)90417-0
[7] https://www.nature.com/articles/s41560-021-00787-9
[8] By “chemical junk” we refer to electrochemical decomposition by-products.
[9] https://pubs.rsc.org/en/content/articlelanding/2017/ta/c7ta00371d
[10] https://doi.org/10.1002/advs.202003675
[11] https://www.nature.com/articles/s41560-018-0107-2
[12] https://www.nature.com/articles/nmat3793
[13] https://pubs.acs.org/doi/full/10.1021/acsenergylett.1c01352
Forward-Looking Statements
This article contains forward-looking statements within the meaning of the federal securities laws and information based on management’s current expectations as of the date of this current report. All statements other than statements of historical fact contained in this article, including statements regarding the future development of QuantumScape’s battery technology, the anticipated benefits of QuantumScape’s technologies and the performance of its batteries, and plans and objectives for future operations, are forward-looking statements. When used in this current report, the words “may,” “will,” “estimate,” “pro forma,” “expect,” “plan,” “believe,” “potential,” “predict,” “target,” “should,” “would,” “could,” “continue,” “believe,” “project,” “intend,” “anticipates” the negative of such terms and other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain such identifying words.
These forward-looking statements are based on management’s current expectations, assumptions, hopes, beliefs, intentions, and strategies regarding future events and are based on currently available information as to the outcome and timing of future events. These forward-looking statements involve significant risks and uncertainties that could cause the actual results to differ materially from the expected results. Many of these factors are outside QuantumScape’s control and are difficult to predict. QuantumScape cautions readers not to place undue reliance upon any forward-looking statements, which speak only as of the date made. Except as otherwise required by applicable law, QuantumScape disclaims any duty to update any forward-looking statements. Should underlying assumptions prove incorrect, actual results and projections could differ materially from those expressed in any forward-looking statements. Additional information concerning these and other factors that could materially affect QuantumScape’s actual results can be found in QuantumScape’s periodic filings with the SEC. QuantumScape’s SEC filings are available publicly on the SEC’s website at www.sec.gov.

I took on the CEO role last year because I see QuantumScape as the global leader in solid state battery technologies with an ability to revolutionize energy storage and create tremendous shareholder value in the process.
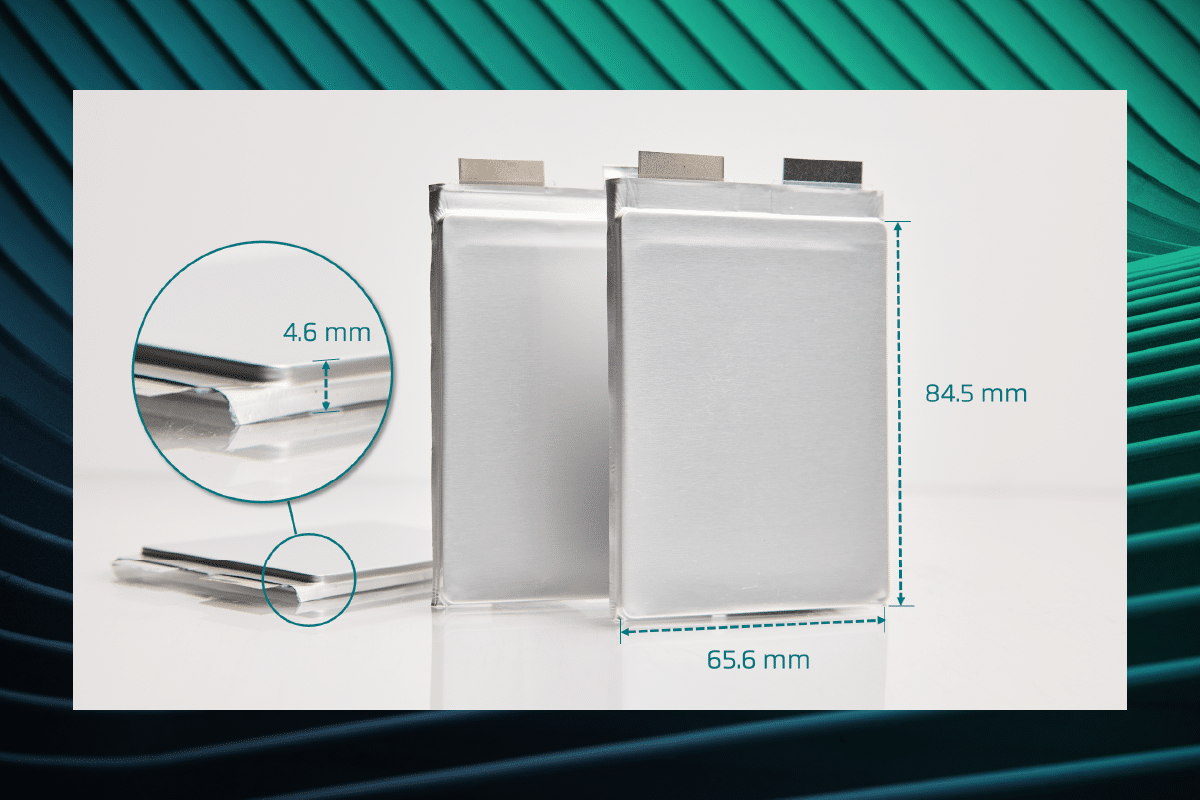
QuantumScape’s planned first commercial product, QSE-5, is a ~5 amp-hour cell designed to meet the requirements of automotive applications. Here, we’ll walk through the various elements of the cell specifications and explain some of the complexities behind the seemingly simple metric of energy density.

I took on the CEO role last year because I see QuantumScape as the global leader in solid state battery technologies with an ability to revolutionize energy storage and create tremendous shareholder value in the process.

QuantumScape’s planned first commercial product, QSE-5, is a ~5 amp-hour cell designed to meet the requirements of automotive applications. Here, we’ll walk through the various elements of the cell specifications and explain some of the complexities behind the seemingly simple metric of energy density.
Privacy Policy | Terms of Use
© 2025 QuantumScape Battery, Inc.
1730 Technology Drive, San Jose, CA 95110
info@quantumscape.com

Pamela Fong is QuantumScape’s Chief of Human Resources Operations, leading people strategy and operations, including talent acquisition, organizational development and employee engagement. Prior to joining the company, Ms. Fong served as the Vice President of Global Human Resources at PDF Solutions (NASDAQ: PDFS), a semiconductor SAAS company. Before that, she served in several HR leadership roles at Foxconn Interconnect Technology, Inc., a multinational electronics manufacturer, and NUMMI, an automotive manufacturing joint venture between Toyota and General Motors. Ms. Fong holds a B.S. in Business Administration from U.C. Berkeley and a M.S. in Management from Stanford Graduate School of Business.